
Leave a Message
We will call you back soon!
 Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters!
 Please check your E-mail!
Please check your E-mail!


NMR allows three-dimensional structural and dynamic information to be obtained in conditions as close as possible to physiological ones. Functional processes can be followed in living cells, and transient protein-protein interactions can be investigated.
What is NMR spectroscopy, and how does it work?
NMR spectroscopy works using the natural magnets—the nuclei of certain atoms—inside proteins. Those natural cellular magnets interact with a big magnet inside the NMR machine. The big magnet forces the protein’s magnets to line up. Researchers then blast the sample with a series of split-second radio-wave pulses and observe how the protein’s magnets respond. Scientists use several sets of these NMR blasts and combine the data to get a more complete picture of the protein. Although X-ray crystallography can examine larger proteins than NMR is able to do, NMR technology can study proteins immersed in liquid solutions. In contrast, X-ray crystallography requires that proteins be organized into crystals.
Introduction:
Nuclear Magnetic Resonance (NMR) has evolved as the main technique to obtain structural information at atomic resolution in solution on macrobiomolecules such as proteins and nucleic acids. Nowadays, solution NMR is an indispensable enabling technology for determining not only structures of such molecules but also their interactions, even weak and transient, as well as for characterising functional processes in solution and also directly in living cells. The power of NMR resides in linking structural, dynamic, kinetic and thermodynamic information so as to make it a technique of choice in cutting-edge research in medicine and biology.
Solution NMR:
Solution NMR within Instruct provides a major approach to obtaining the molecular-level information needed to build networks of interactions responsible for vital cellular processes, and to describe them at molecular level. Thanks to the recent hardware and software developments, its applicability ranges from supra-molecular structures to intrinsically unfolded proteins at almost physiological concentrations. Solution NMR offers unique possibilities to study dynamic processes at atomic resolution and over a very wide range of timescales, from picoseconds to hours, including folding mechanisms and transient formation of complexes.
Solid State NMR:
While solution NMR is already a well-established technique for the structural determination of biomolecules in solution, solid state (SS) NMR has experienced tremendous methodological and technical advancements over the last decade, and is reaching the status of a powerful technique for the mechanistic and structural investigation of biological solids. SS NMR is intrinsically free of the limitations imposed on liquid state NMR by the size of the system under investigation, and can handle molecular systems that are not amenable to X-ray studies such as insoluble aggregates and fibrils. New exciting possibilities are discovered almost daily and SS NMR is thus expected to open new avenues for modern biology in the near future.
SS NMR within Instruct provides an invaluable tool for the determination of the structure and dynamics of systems that are beyond the reach of other structural methods. It has a wide applicability, ranging from membrane proteins to nano-crystalline materials to insoluble aggregates and fibrils. State-of-the-art instruments and experimental protocols enable the determination of a number of biophysical parameters allowing, along with structural determination, the characterization of both the internal and global dynamics of the system at atomic detail. These features make SS NMR a vital technique in structural biology.
Fast field cycling relaxometry:
Fast field cycling relaxometry is a tool for measuring nuclear relaxation rates from very low magnetic fields (0.01 MHz proton Larmor frequency) up to 1 T (about 45 MHz proton Larmor frequency). The field dependence of the relaxation rates provides information on the structural and dynamic features of the molecule and, in the case of paramagnetic systems, on the electron relaxation.
Relaxometry measurements are usually performed in water solutions. They provide information on the correlation times modulating the dipolar interactions between protons, and thus on the reorientation time and aggregation state of the system. Relaxometry measurements can be useful in determining the presence of binding between macromolecules or between a small paramagnetic complex and a macromolecule, as well as for studying the mechanisms responsible for electron relaxation. It is largely used for the characterisation of contrast agents for magnetic resonance imaging (MRI) and for their optimization. This technique is also successfully applied to the characterization of radicals for applications to dynamic nuclear polarization (DNP).
Electron Paramagnetic Resonance (EPR):
Electron Paramagnetic Resonance (EPR) measures the absorption of electromagnetic radiation by a paramagnetic system placed in a static magnetic field. Standard applications of EPR include characterisation of free radicals, studies of reactions involving radicals/paramagnetic metals, and investigations of the electronic and structural properties of paramagnetic centers. In complex biological systems with stable or transient paramagnetic centres (which can be metal ions or clusters, spin labels, amino acid radicals, or organic cofactor radicals) EPR is used to study the arrangement of cofactors and subunits, the formation of secondary structure elements, or the interactions between biomolecules.
The information obtained is used in the determination of molecular structure. The accuracy of this structural information often exceeds that of other methods. In many cases, EPR spectroscopic data provide the only structural information, in particular when high-resolution crystals are not available and systems are too large for high-resolution NMR spectroscopy. EPR data can complement the information gained by other structural methodologies, and has proved to be an essential technique for interdisciplinary investigations of biological systems.
What can we do with NMR:
KS-V Peptide NMR Analysis Services Platform:
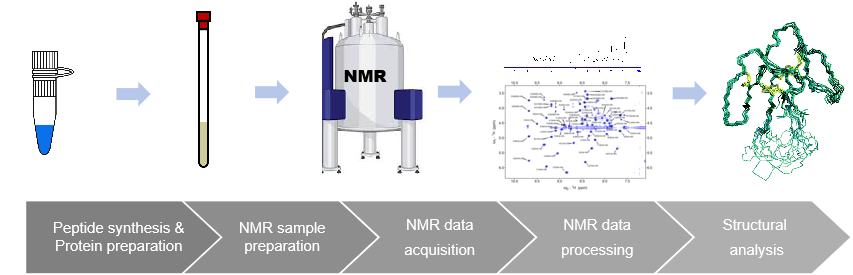


NMR allows three-dimensional structural and dynamic information to be obtained in conditions as close as possible to physiological ones. Functional processes can be followed in living cells, and transient protein-protein interactions can be investigated.
What is NMR spectroscopy, and how does it work?
NMR spectroscopy works using the natural magnets—the nuclei of certain atoms—inside proteins. Those natural cellular magnets interact with a big magnet inside the NMR machine. The big magnet forces the protein’s magnets to line up. Researchers then blast the sample with a series of split-second radio-wave pulses and observe how the protein’s magnets respond. Scientists use several sets of these NMR blasts and combine the data to get a more complete picture of the protein. Although X-ray crystallography can examine larger proteins than NMR is able to do, NMR technology can study proteins immersed in liquid solutions. In contrast, X-ray crystallography requires that proteins be organized into crystals.
Introduction:
Nuclear Magnetic Resonance (NMR) has evolved as the main technique to obtain structural information at atomic resolution in solution on macrobiomolecules such as proteins and nucleic acids. Nowadays, solution NMR is an indispensable enabling technology for determining not only structures of such molecules but also their interactions, even weak and transient, as well as for characterising functional processes in solution and also directly in living cells. The power of NMR resides in linking structural, dynamic, kinetic and thermodynamic information so as to make it a technique of choice in cutting-edge research in medicine and biology.
Solution NMR:
Solution NMR within Instruct provides a major approach to obtaining the molecular-level information needed to build networks of interactions responsible for vital cellular processes, and to describe them at molecular level. Thanks to the recent hardware and software developments, its applicability ranges from supra-molecular structures to intrinsically unfolded proteins at almost physiological concentrations. Solution NMR offers unique possibilities to study dynamic processes at atomic resolution and over a very wide range of timescales, from picoseconds to hours, including folding mechanisms and transient formation of complexes.
Solid State NMR:
While solution NMR is already a well-established technique for the structural determination of biomolecules in solution, solid state (SS) NMR has experienced tremendous methodological and technical advancements over the last decade, and is reaching the status of a powerful technique for the mechanistic and structural investigation of biological solids. SS NMR is intrinsically free of the limitations imposed on liquid state NMR by the size of the system under investigation, and can handle molecular systems that are not amenable to X-ray studies such as insoluble aggregates and fibrils. New exciting possibilities are discovered almost daily and SS NMR is thus expected to open new avenues for modern biology in the near future.
SS NMR within Instruct provides an invaluable tool for the determination of the structure and dynamics of systems that are beyond the reach of other structural methods. It has a wide applicability, ranging from membrane proteins to nano-crystalline materials to insoluble aggregates and fibrils. State-of-the-art instruments and experimental protocols enable the determination of a number of biophysical parameters allowing, along with structural determination, the characterization of both the internal and global dynamics of the system at atomic detail. These features make SS NMR a vital technique in structural biology.
Fast field cycling relaxometry:
Fast field cycling relaxometry is a tool for measuring nuclear relaxation rates from very low magnetic fields (0.01 MHz proton Larmor frequency) up to 1 T (about 45 MHz proton Larmor frequency). The field dependence of the relaxation rates provides information on the structural and dynamic features of the molecule and, in the case of paramagnetic systems, on the electron relaxation.
Relaxometry measurements are usually performed in water solutions. They provide information on the correlation times modulating the dipolar interactions between protons, and thus on the reorientation time and aggregation state of the system. Relaxometry measurements can be useful in determining the presence of binding between macromolecules or between a small paramagnetic complex and a macromolecule, as well as for studying the mechanisms responsible for electron relaxation. It is largely used for the characterisation of contrast agents for magnetic resonance imaging (MRI) and for their optimization. This technique is also successfully applied to the characterization of radicals for applications to dynamic nuclear polarization (DNP).
Electron Paramagnetic Resonance (EPR):
Electron Paramagnetic Resonance (EPR) measures the absorption of electromagnetic radiation by a paramagnetic system placed in a static magnetic field. Standard applications of EPR include characterisation of free radicals, studies of reactions involving radicals/paramagnetic metals, and investigations of the electronic and structural properties of paramagnetic centers. In complex biological systems with stable or transient paramagnetic centres (which can be metal ions or clusters, spin labels, amino acid radicals, or organic cofactor radicals) EPR is used to study the arrangement of cofactors and subunits, the formation of secondary structure elements, or the interactions between biomolecules.
The information obtained is used in the determination of molecular structure. The accuracy of this structural information often exceeds that of other methods. In many cases, EPR spectroscopic data provide the only structural information, in particular when high-resolution crystals are not available and systems are too large for high-resolution NMR spectroscopy. EPR data can complement the information gained by other structural methodologies, and has proved to be an essential technique for interdisciplinary investigations of biological systems.
What can we do with NMR:
KS-V Peptide NMR Analysis Services Platform:
