
Leave a Message
We will call you back soon!
 Your message must be between 20-3,000 characters!
Your message must be between 20-3,000 characters!
 Please check your E-mail!
Please check your E-mail!
Biological processes are regulated by the interaction of molecules through specific molecular contacts, forming stable yet irreversible complexes. These interactions are highly determined by the principles of thermodynamics as well as biomolecular structure and recognition. Thus, finding the binding site and quantifying the strength (i.e., binding affinity) interaction is essential to understanding biological processes, and designing of drugs.
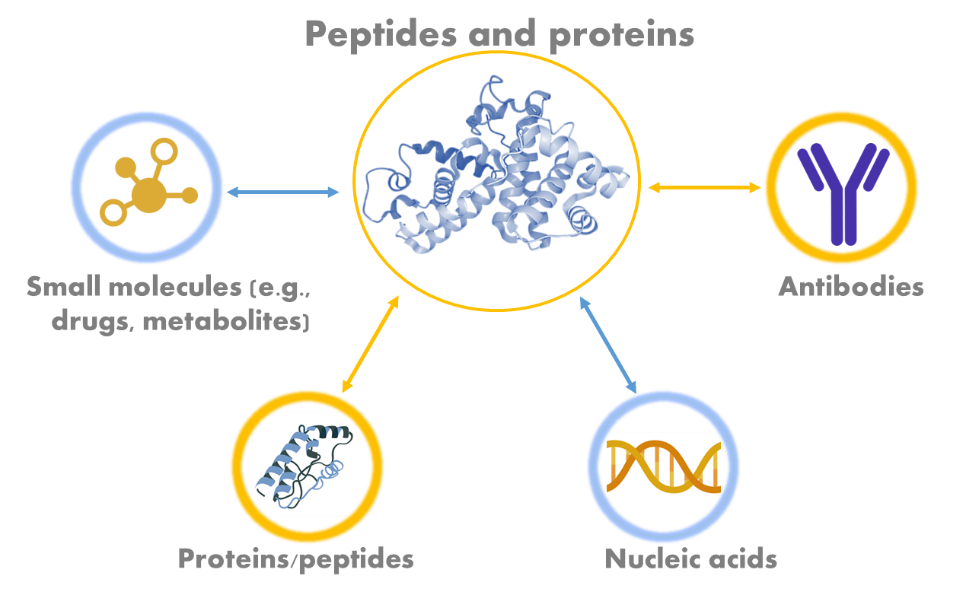
Biomolecular interaction analysis, often referred to as BIA, is a scientific method used to study and analyze the interactions between biomolecules, such as proteins, nucleic acids, and small molecules, in order to better understand their binding affinities, kinetics, and other important characteristics. This technique is fundamental in fields like biochemistry, molecular biology, and drug discovery, as it provides insights into the mechanisms and strengths of molecular interactions. BIA is widely applied in drug screening and development due to its focus on the quantification of interactions between biomolecules. The outcome of such detailed studies help us understand how potential agonists or antagonists interact with a drug target, as well as quantify the binding affinity of a ligand to its receptor.
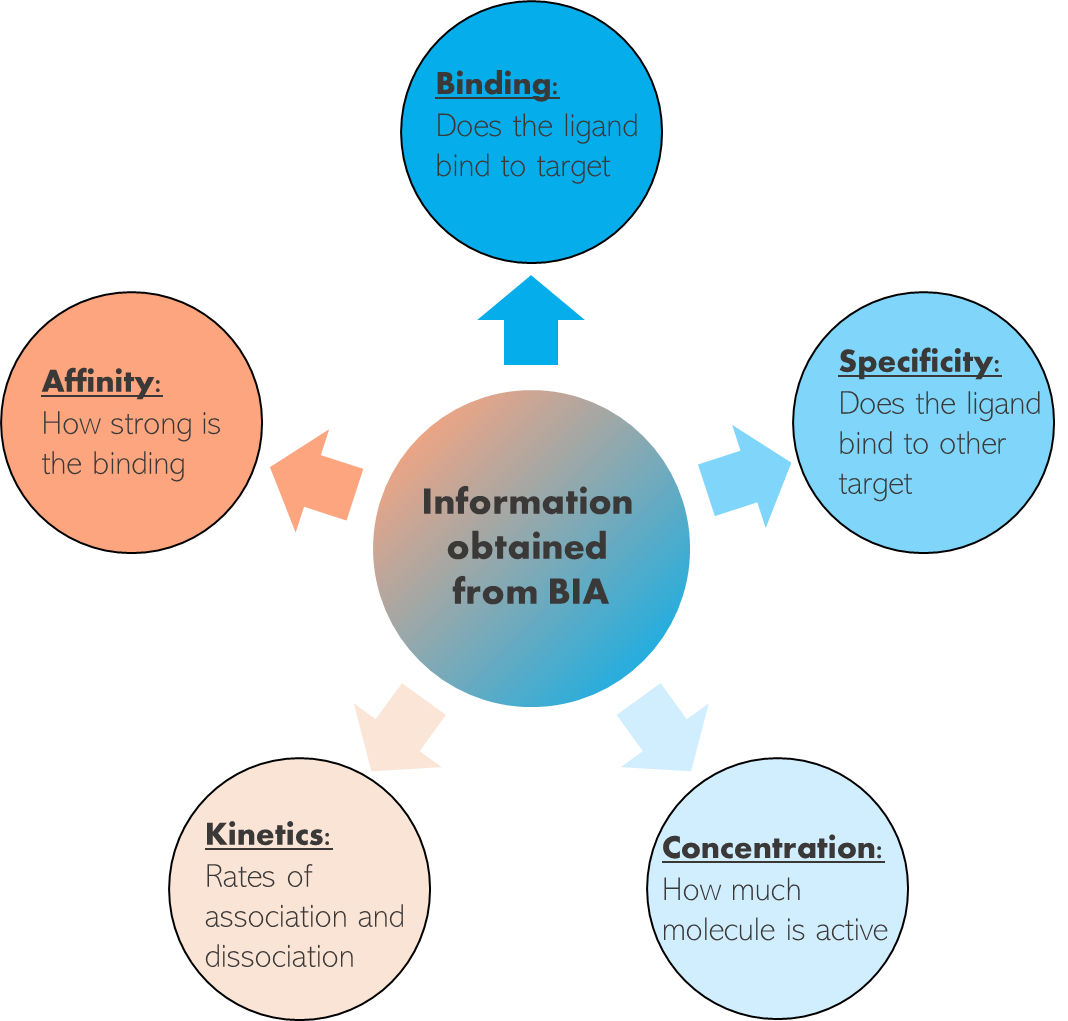
The primary technique used in biomolecular interaction analysis is surface plasmon resonance (SPR). SPR is a label-free and real-time analytical technique that allows researchers to monitor the binding between molecules without the need for fluorescent or radioactive labels. Here's how it works:
Apart from SPR, other techniques and instruments used in biomolecular interaction analysis include:
These techniques and instruments enable researchers to gain insights into the fundamental aspects of molecular interactions, which is critical for understanding biological processes, developing new drugs, and advancing fields like structural biology, immunology, and molecular genetics.

Biological processes are regulated by the interaction of molecules through specific molecular contacts, forming stable yet irreversible complexes. These interactions are highly determined by the principles of thermodynamics as well as biomolecular structure and recognition. Thus, finding the binding site and quantifying the strength (i.e., binding affinity) interaction is essential to understanding biological processes, and designing of drugs.

Biomolecular interaction analysis, often referred to as BIA, is a scientific method used to study and analyze the interactions between biomolecules, such as proteins, nucleic acids, and small molecules, in order to better understand their binding affinities, kinetics, and other important characteristics. This technique is fundamental in fields like biochemistry, molecular biology, and drug discovery, as it provides insights into the mechanisms and strengths of molecular interactions. BIA is widely applied in drug screening and development due to its focus on the quantification of interactions between biomolecules. The outcome of such detailed studies help us understand how potential agonists or antagonists interact with a drug target, as well as quantify the binding affinity of a ligand to its receptor.

The primary technique used in biomolecular interaction analysis is surface plasmon resonance (SPR). SPR is a label-free and real-time analytical technique that allows researchers to monitor the binding between molecules without the need for fluorescent or radioactive labels. Here's how it works:
Apart from SPR, other techniques and instruments used in biomolecular interaction analysis include:
These techniques and instruments enable researchers to gain insights into the fundamental aspects of molecular interactions, which is critical for understanding biological processes, developing new drugs, and advancing fields like structural biology, immunology, and molecular genetics.